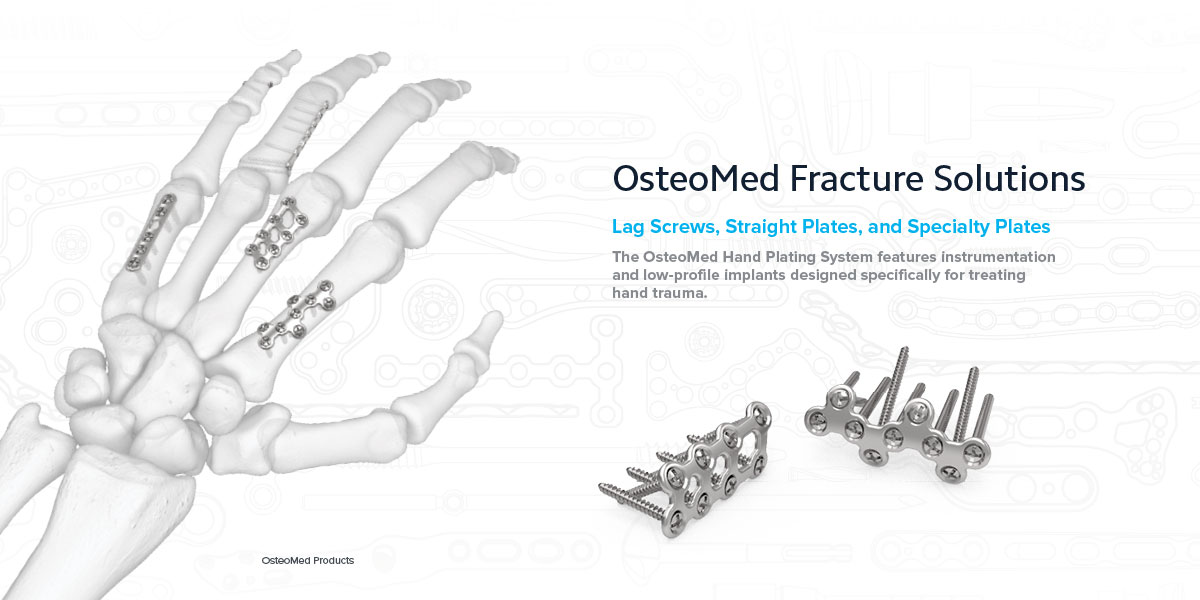
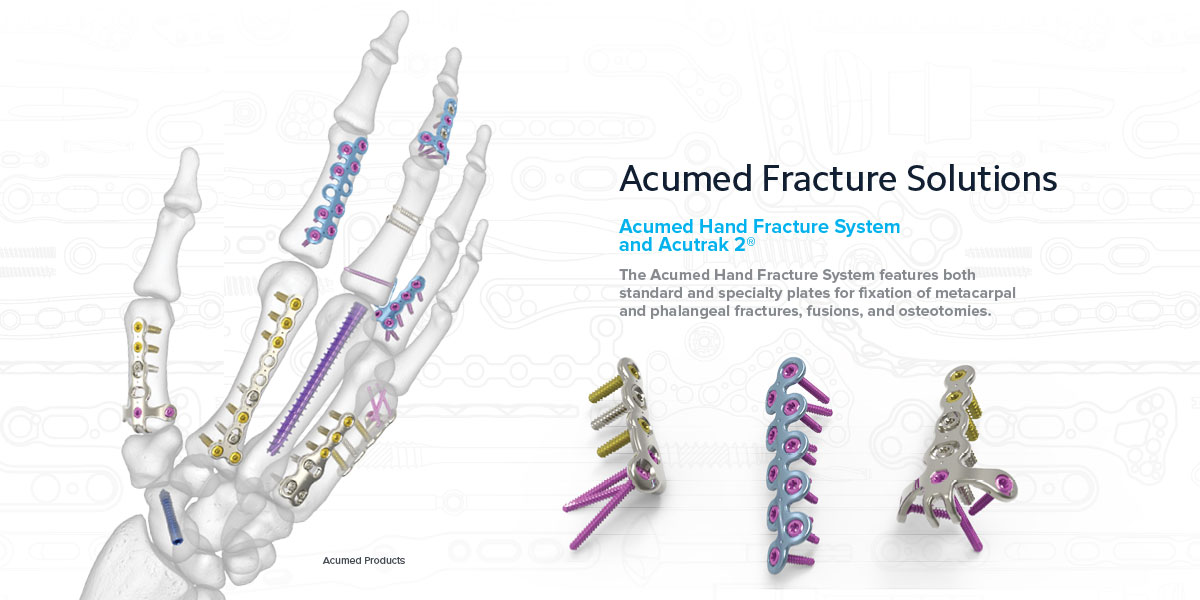
Fracture Solutions
OsteoMed Hand Plating System
Lag Screws 1.2 mm Lag Screws capture small avulsed fragments.
Straight Plates Like T and Y Plates, these may be cut to length and bent to better fit patient anatomy.
Offset Grid Plate Designed to provide fixation of comminuted diaphyseal fractures.
The OsteoMed Hand Plating System features 68 plates, 4 screw types, and 3 k-wires.

| Modules | Distal Phalanges | Middle Phalanges | Proximal Phalanges | Metacarpals and Carpals |
|---|---|---|---|---|
| 1.2 mm | ||||
| 1.6 mm | ||||
| 2.0 mm | ||||
| 2.4 mm |

TaperLock™ Screw Retention Technology
Acumed Hand Fracture System
Metacarpal Neck Fracture: The 1.3 mm Metacarpal Neck Plate has three distally pointing converging screws to provide metacarpal head fixation.
Rotational Malunion Osteotomy: The 1.3 mm Rotational Correction Plate system includes a Rotational Osteotomy Cutting Guide designed to facilitate placement and orientation of the cut for rotational osteotomies of the metacarpals.
Dorsal Scaphoid Fracture: The Acutrak 2® Headless Compression Screw is designed to effectively reduce and secure a fractured scaphoid and maintain rotational stability.
The Acumed Hand Fracture System offers plates in 0.8 mm & 1.3 mm thicknesses.

Rolando Fracture Hook Plate
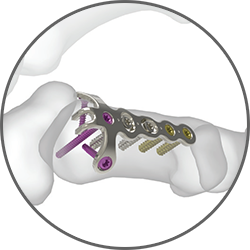 The 1.3 mm Rolando Fracture Hook Plate is designed for a three-part fracture pattern at the base of the first metacarpal. The prongs on the proximal end of the plate should contact the dorsal surface of the abductor pollicis longus (APL) tendon and support any comminution of the base of the first metacarpal.
The 1.3 mm Rolando Fracture Hook Plate is designed for a three-part fracture pattern at the base of the first metacarpal. The prongs on the proximal end of the plate should contact the dorsal surface of the abductor pollicis longus (APL) tendon and support any comminution of the base of the first metacarpal.Avulsion Hook Plate
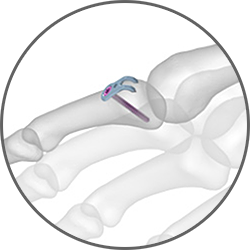 The 0.8 mm Avulsion Hook Plate is designed to provide more stability than a K-wire when a fragment is too small for a single lag screw. The plate’s prongs can support an avulsion fragment when the fragment is too small for a lag screw and more stability is desired than pinning with K-wires alone can provide.
The 0.8 mm Avulsion Hook Plate is designed to provide more stability than a K-wire when a fragment is too small for a single lag screw. The plate’s prongs can support an avulsion fragment when the fragment is too small for a lag screw and more stability is desired than pinning with K-wires alone can provide.Hand Fracture System Cadaveric Lab Part 2: Rolando Fracture Hook Plate
Hand Fracture System Cadaveric Lab Part 1: System Overview and Design Rationale
Hand Fracture System Cadaveric Lab Part 4: Avulsion Hook Plate
Hand Fracture System Cadaveric Lab Part 3: Curved Medial Lateral Plate
Key Publications
Case Studies
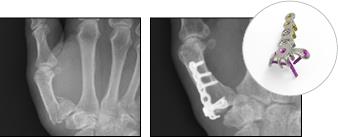
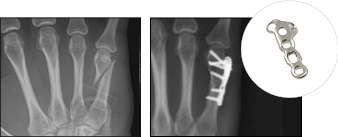
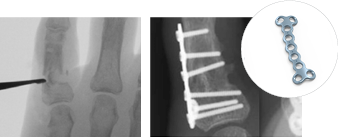
GFS-00123-01 Data on file at Vivorté
*Competitive data on file with Acumed.
1. Ozyurekoglu T, Turker T. Results of a method of 4-corner arthrodesis using headless compression screws. J Hand Surg Am. 2012;37(3):486–492.
These materials contain information about products that may or may not be available in any particular country or may be available under different trademarks in different countries. The products may be approved or cleared by governmental regulatory organizations for sale or use with different indications or restrictions in different countries. Products may not be approved for use in all countries. Nothing contained in these materials should be construed as a promotion or solicitation for any product or for the use of any product in a particular way that is not authorized under the laws and regulations of the country where the reader is located. Nothing in these materials should be construed as a representation or warranty as to the efficacy or quality of any product, nor the appropriateness of any product to treat any specific condition. Physicians may direct questions about the availability and use of the products described in these materials to their authorized Acumed distributor. Specific questions patients may have about the use of the products described in these materials or the appropriateness for their own conditions should be directed to their own physician.
Acumed® and Acutrak 2® are registered trademarks of Acumed LLC.
OsteoMed® and TaperLock™ are registered trademarks of OsteoMed LLC.
Vivorté®, Trabexus®, and, Fortera® are registered trademarks of Vivorté.