A Complete Range of Solutions for Hand Fractures
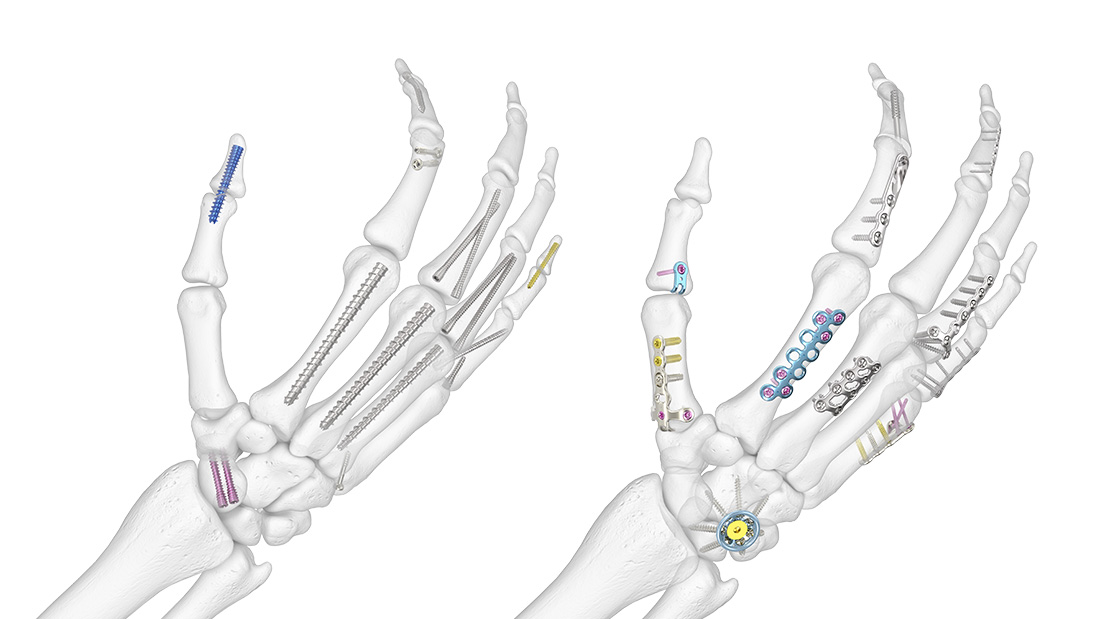
Hand Fracture Solutions That Have You Covered
OsteoMed Hand Fusion System Surgical Technique Lab with Dr. William Geissler
Perspectives on PIP & MCP Fusions with Dr William Geissler
Surgical Approach to Rolando Fractures with Asif M Ilyas, MD
Acutrak 3 Headless Compression Screw System Overview
Hand Fracture System Overview
OsteoMed Solutions
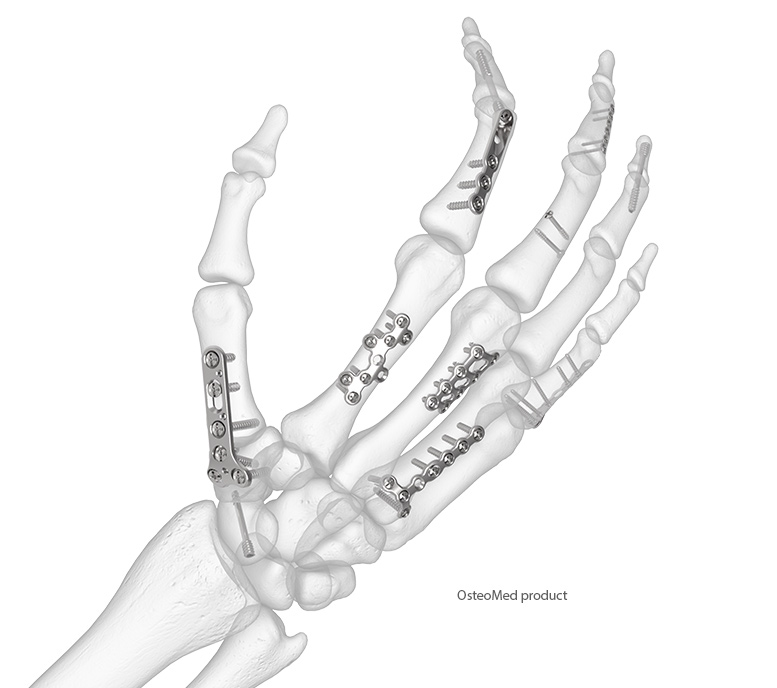
The OsteoMed Hand Plating System (HPS) features a full range of low-profile plates in four size modules: 1.2, 1.6, 2.0, and 2.4 mm. The HPS Plates accept up to four screw types: VA locking, non-locking, lag, and cannulated. Also featured are hand fusion plates in two different sizes. The HPS highlights include:
- A full range with 68 plates with 4 screw diameters
- Specialty implants for CMC, PIP, and MCP fusions
- TaperLock Technology – Allows for a low-profile screw and plate construct
- Plates with dual compression holes
- Cannulated compression screws in 2.0, 2.4, and 3.0 mm diameters
Acumed Solutions
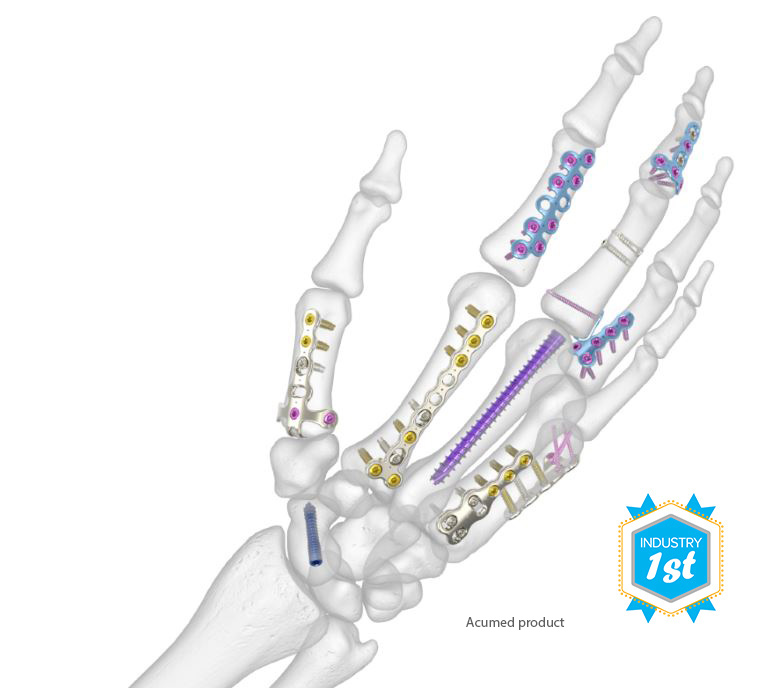
The Acumed® Hand Fracture System is designed to surgically treat metacarpal and phalangeal fractures, fusions, and osteotomies, and includes five solutions in one tray.
Hand Fracture System highlights include:
- Precontoured standard and specialty plates
- Acumed’s Hexalobe MultiScrew technology eliminates the need for traditional locking and nonlocking screws
- Specialized instruments, including an osteotomy cutting guide, the SaveLoc compression sleeve, unique clamps and forceps, and a plate cutter
- The Acumed Small Bone Fixator and Small Bone Distractor provide external fixation and bone lengthening options
An Industry First: Hand Fracture System
Launched in 2014, these are the first hand plates that accept multiple Hand Fracture System screw diameters in every hole of every plate in the system.
| Indication | OsteoMed | Acumed |
|---|---|---|
| Avulsion Fracture | 1.2 mm Screw | Avulsion Hook Plate |
| Boxer’s Fracture | Subcondylar Plate | Metacarpal Neck Fracture Plate |
| Transverse Fracture | Straight Plate, T Plate, L/R Plate | Straight Plate, T Plate, Medial/Lateral Plate |
| Short Oblique | Cannulated Lag Screws | Headless Compression Screws |
| Comminuted/Long Oblique | Z Plate, Offset Grid Plate | Offset Plate |
| Fusion | PIP/CMC Fusion Plate | Acutrak 2® Headless Compression Screws |
| Malunion | - | Rotational Correction Plate |
| Rolando/Bennett | - | Rolando Fracture Hook Plate |
| Acumed Standard Plates | Thickness | Length |
|---|---|---|
| Compression Plate, 6-hole | 0.8 mm | 32.3 mm |
| Compression Plate, 6-hole | 1.3 mm | 38.3 mm |
| Straight Plate, 10-hole | 0.8 mm | 50.2 mm |
| Straight Plate, 10-hole | 1.3 mm | 60.2 mm |
| T-Plate | 0.8 mm | 50.0 mm |
| T-Plate | 1.3 mm | 59.9 mm |
| Offset Plate | 0.8 mm | 35.0 mm |
| Acumed Specialty Plates | Thickness | Length |
|---|---|---|
| Curved Medial/Lateral Plate | 0.8 mm | 35.8 mm |
| Avulsion Hook Plate | 0.8 mm | 10.0 mm |
| Metacarpal Neck Plate, Left & Right | 1.3 mm | 27.8 mm |
| Rolando Fracture Hook Plate | 1.3 mm | 34.6 mm |
| Rotational Correction Plate | 1.3 mm | 33.7 mm |
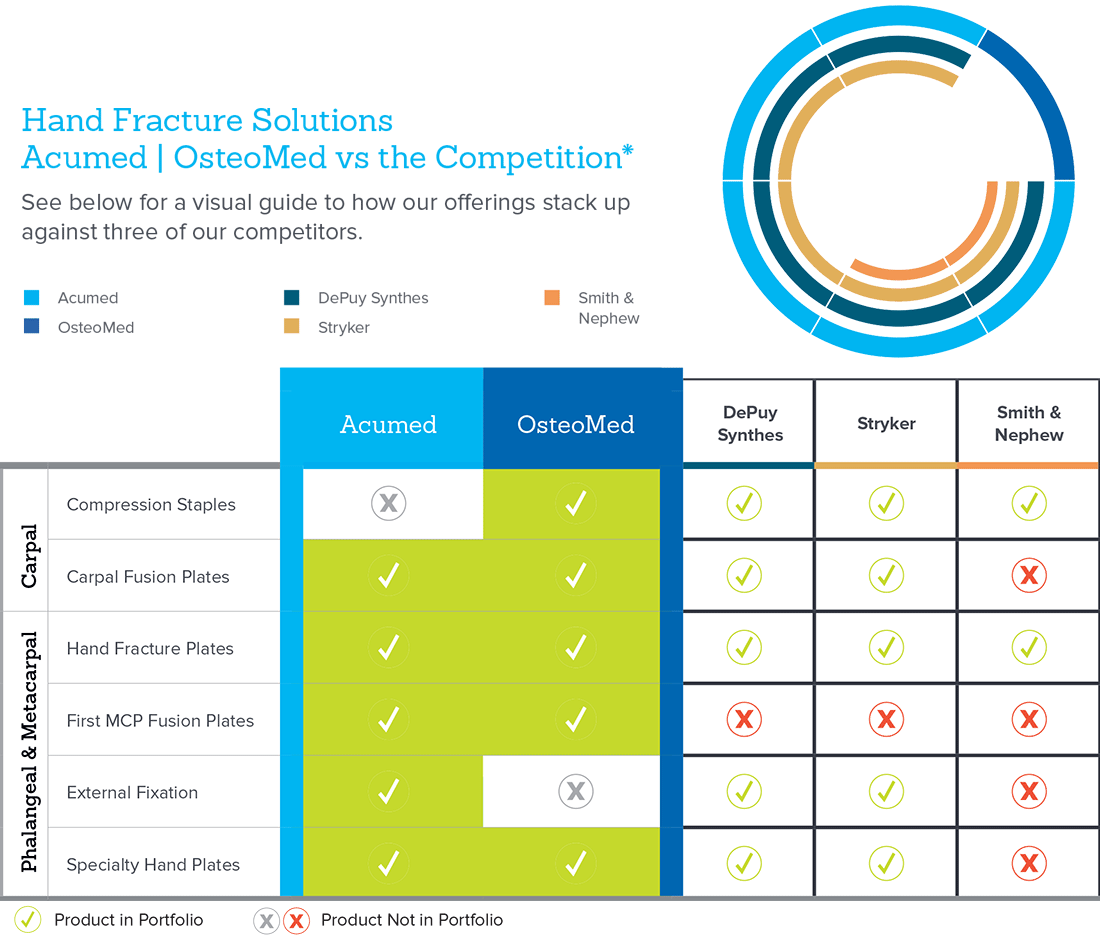
Hand Fracture Solutions
GFS-00123-01 Data on file at Vivorté
*Competitive data on file with Acumed.
1. Ozyurekoglu T, Turker T. Results of a method of 4-corner arthrodesis using headless compression screws. J Hand Surg Am. 2012;37(3):486–492.
These materials contain information about products that may or may not be available in any particular country or may be available under different trademarks in different countries. The products may be approved or cleared by governmental regulatory organizations for sale or use with different indications or restrictions in different countries. Products may not be approved for use in all countries. Nothing contained in these materials should be construed as a promotion or solicitation for any product or for the use of any product in a particular way that is not authorized under the laws and regulations of the country where the reader is located. Nothing in these materials should be construed as a representation or warranty as to the efficacy or quality of any product, nor the appropriateness of any product to treat any specific condition. Physicians may direct questions about the availability and use of the products described in these materials to their authorized Acumed distributor. Specific questions patients may have about the use of the products described in these materials or the appropriateness for their own conditions should be directed to their own physician.
Acumed® and Acutrak 2® are registered trademarks of Acumed LLC.
OsteoMed® and TaperLock™ are registered trademarks of OsteoMed LLC.
Vivorté®, Trabexus®, and, Fortera® are registered trademarks of Vivorté.