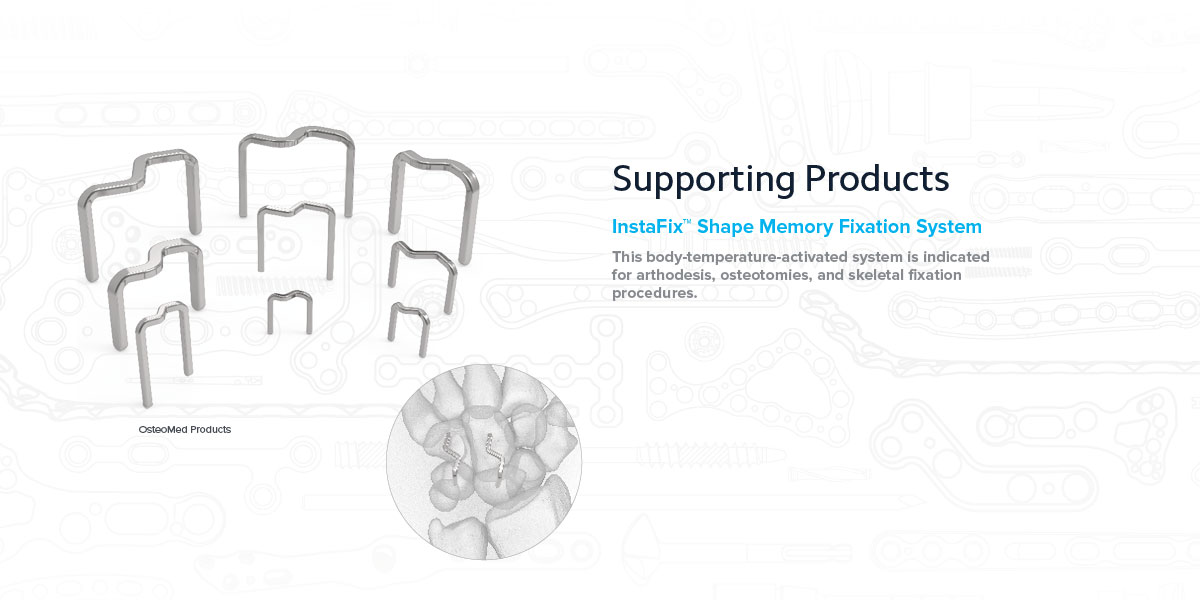
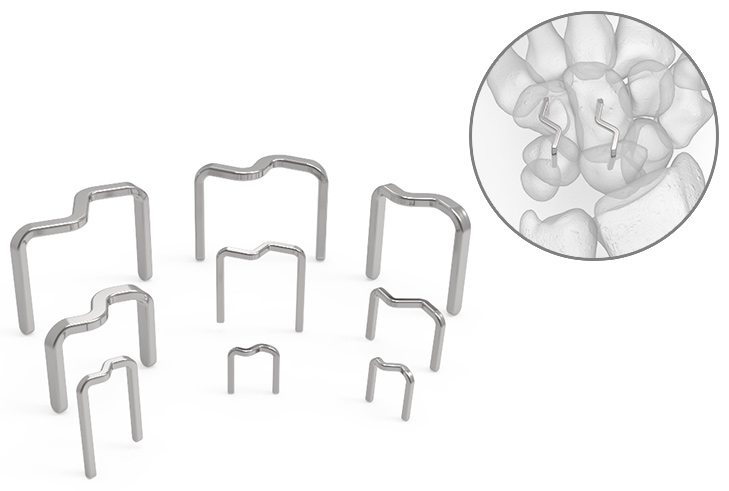
Supporting Products
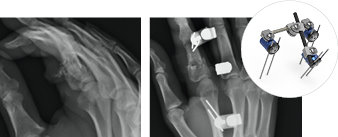
InstaFix™ Shape Memory Fixation System
Low-profile design helps minimize tissue disruption
An S-back design provides continuous and active bicortical compression at the surgical site.
InstaFix is packaged sterile with fully disposable instrumentation, and includes sizes from 8x8 mm to 30x20 mm to accommodate a range of procedures
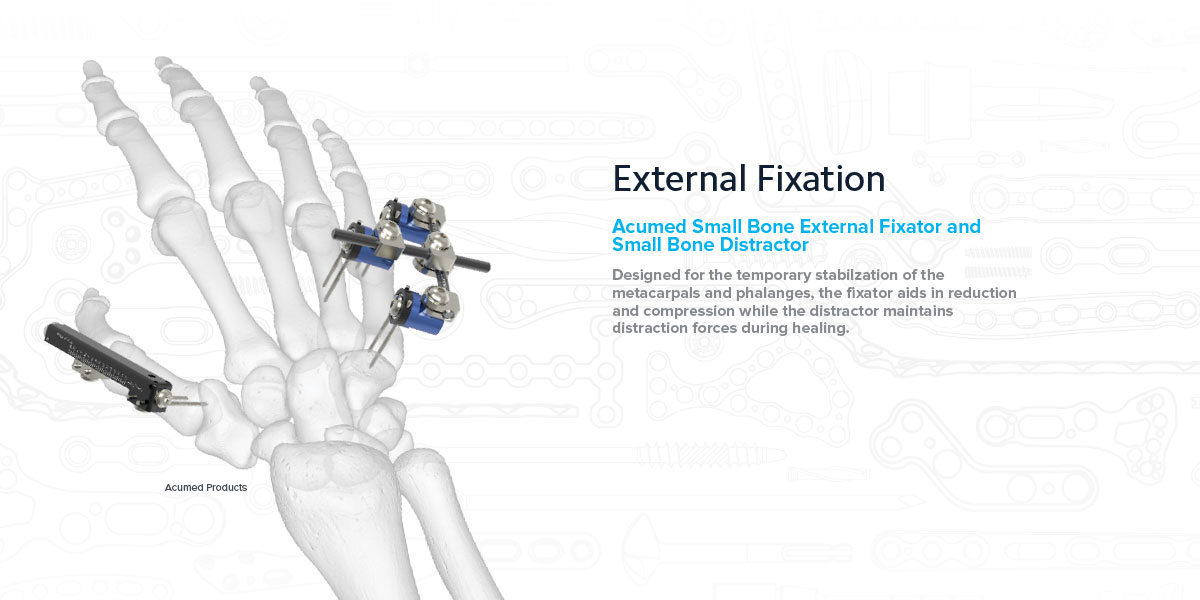
A Pair of Solutions for External Fixation
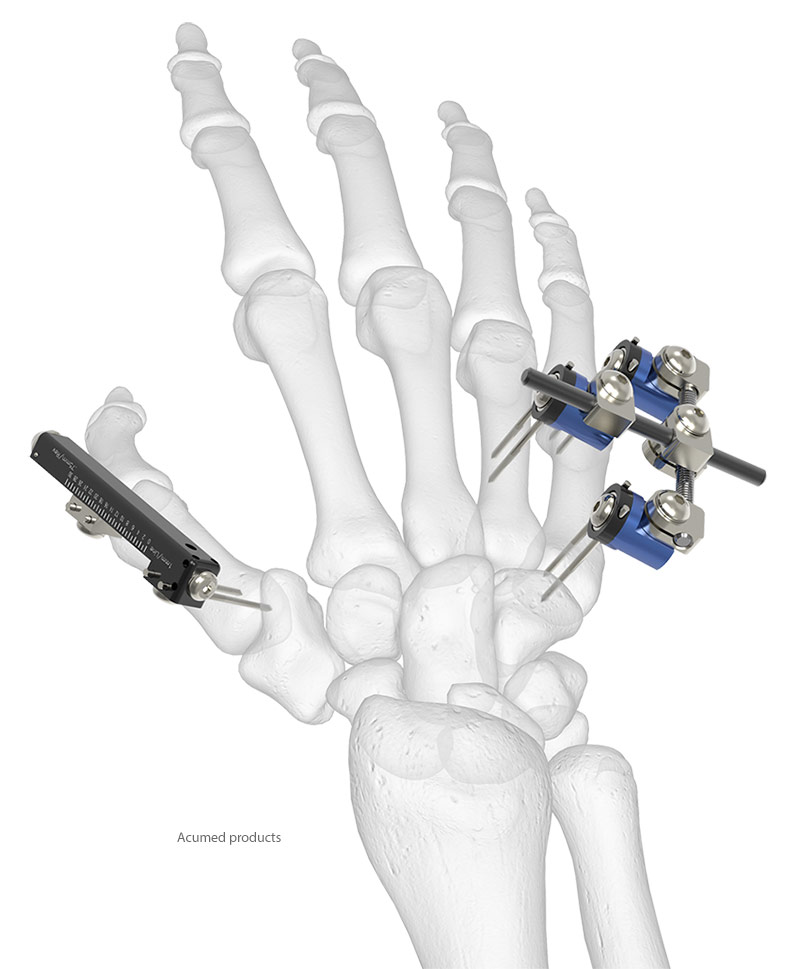
Acumed Small Bone External Fixator
Acumed Small Bone Distractor
Case Study
Orthobiologics / Bone Void Fillers

OsteoMed Magnesium Phosphate Bone Void Filler
- Enhanced remodeling – 80% in 26 weeks
- Moldable or Injectable, delivery and mixing options

Vivorté® Trabexus™ Osteoinductive Calcium Phosphate Bone Void Filler
- Induces recruitment of bone forming cells to the implant site.**
- Can be placed manually or extruded through the cannula provided.
- Isothermically sets at 15 minutes.
Vivorté® Fortera™ Osteoconductive Calcium Phosphate Bone Void Filler
- Injectable through a 16G cannula
- Six minutes of handling time after mixing
- Room temperature storage
- Isothermically sets at 15 minutes
OsteoMed Amniotic Membrane
- Used as a covering for reconstructive procedures
- Offer protection from surrounding environment
Vivorté Trabexus Mixing Instructions
Trabexus or Trabecular Osteoinductive Biocement is a self-setting calcium phosphate matrix with engineered allograft bone particles for optimized compressive strength and resorptive characteristics. Mixing instructions for Trabexus are detailed in this video. Acumed is the exclusive distributor for Trabexus. Click here for more information from the manufacturer.
Vivorté Fortera Mixing Instructions
GFS-00123-01 Data on file at Vivorté
*Competitive data on file with Acumed.
1. Ozyurekoglu T, Turker T. Results of a method of 4-corner arthrodesis using headless compression screws. J Hand Surg Am. 2012;37(3):486–492.
These materials contain information about products that may or may not be available in any particular country or may be available under different trademarks in different countries. The products may be approved or cleared by governmental regulatory organizations for sale or use with different indications or restrictions in different countries. Products may not be approved for use in all countries. Nothing contained in these materials should be construed as a promotion or solicitation for any product or for the use of any product in a particular way that is not authorized under the laws and regulations of the country where the reader is located. Nothing in these materials should be construed as a representation or warranty as to the efficacy or quality of any product, nor the appropriateness of any product to treat any specific condition. Physicians may direct questions about the availability and use of the products described in these materials to their authorized Acumed distributor. Specific questions patients may have about the use of the products described in these materials or the appropriateness for their own conditions should be directed to their own physician.
Acumed® and Acutrak 2® are registered trademarks of Acumed LLC.
OsteoMed® and TaperLock™ are registered trademarks of OsteoMed LLC.
Vivorté®, Trabexus®, and, Fortera® are registered trademarks of Vivorté.